VACCELERATE Italy - We Are Growing!
- Oct 28, 2021
- 1 min read
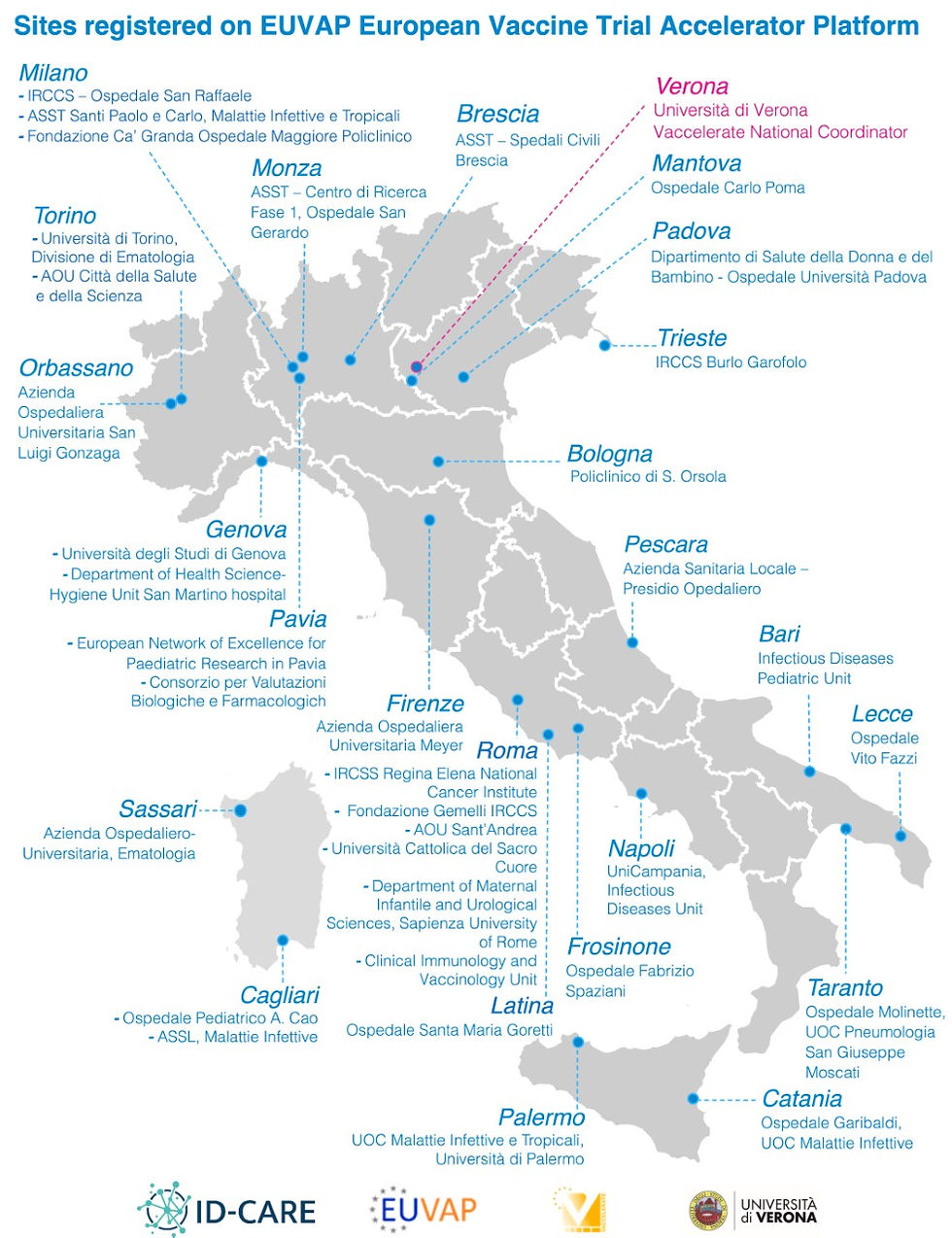
Even more sites all over the country, have joined the EUVAP (European Vaccine Trial Platform). They are now part of a Europe-wide database of 469 experienced clinical trial sites interested in conducting COVID-19 vaccine studies, with a common goal of accelerating clinical development of vaccines for all populations and risk groups.
We are looking forward to the new collaborations that will follow!



Comments